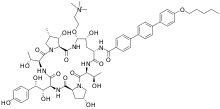
 | |
| Clinical data | |
|---|---|
| Trade names | Rezzayo |
| Other names | Biafungin; CD101 |
| License data |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C63H85N8O17+ |
| Molar mass | 1226.4 g/mol |
Rezafungin (trade name Rezzayo) is an antifungal drug of the echinocandin class.[1]
Rezafungin was approved by the Food and Drug Administration in March 2023 for the treatment of candidemia and invasive candidiasis in adults with limited or no alternative treatment options.[2][3]
References
- ↑ Zhao Y, Perlin DS (September 2020). "Review of the Novel Echinocandin Antifungal Rezafungin: Animal Studies and Clinical Data". Journal of Fungi. 6 (4): 192. doi:10.3390/jof6040192. PMC 7712954. PMID 32998224.
- ↑ "Rezzayo approved by FDA amid rapid Candida auris spread". thepharmaletter.com. March 23, 2023.
- ↑ Syed YY (June 2023). "Rezafungin: First Approval". Drugs. 83 (9): 833–840. doi:10.1007/s40265-023-01891-8. PMID 37212966.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.