| Plant defensin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | Plant defensin | ||||||||
| Pfam | PF00304 | ||||||||
| Pfam clan | CL0054 | ||||||||
| InterPro | IPR008176 | ||||||||
| PROSITE | PDOC00725 | ||||||||
| SCOP2 | 1gps / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 58 | ||||||||
| OPM protein | 1jkz | ||||||||
| CDD | cd00107 | ||||||||
| |||||||||
Plant defensins (formerly gamma-thionins) are a family of small, cysteine-rich defensins found in plants that serve to defend them against pathogens and parasites.[1]
History
The first plant defensins were discovered in barley and wheat in 1990 and were initially designated as a γ-thionins.[2][3] In 1995, the name was changed to 'plant defensin' when it was identified that they are evolutionarily unrelated to other thionins and were more similar to defensins from insects and mammals.[4][5]
Function
Plant defensins are a large component of the plant innate immune system. A plant genome typically contains large numbers of different defensin genes[6] that vary in their efficacies against different pathogens and the amount they are expressed in different tissues.[7]
Antimicrobial activity
The modes of action of different defensins depends on the type of fungus they are interacting with. Most characterized plant defensins are antimicrobial peptides. Both antifungal and antibacterial plant defensins have been identified,[8][9] although their exact mechanisms of action vary.[7]
Enzyme inhibition
Some plant defensins have also been identified as enzyme inhibitors of α-amylase or trypsin.[10][11][12] It is believed that these are antifeedant activities to deter insects.[11]
Anti-cancer
An additional promiscuous activity of some plant defensins is stopping the growth or disrupting the membranes of cancer cells in in vitro experiments.[13][14]
Structure
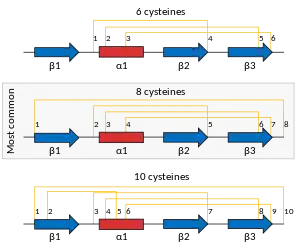
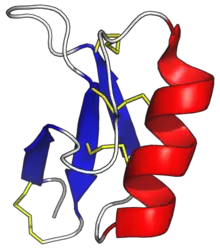
Defensin proteins are produced as a precursor protein with one or two prodomains that are removed to make the final mature protein. In their mature form, they generally consist of about 45 to 50 amino-acid residues. The folded structure is characterised by a well-defined 3-stranded anti-parallel beta-sheet and a short alpha-helix.[15] The structure of most plant defensins is cross-linked by four disulfide bridges: three in core and one linking the N- and C-termini.[1] Some plant defensins have only the core three disulphides, and a few have been found with an additional one (resulting in five total bridges).[16]
Evolution
Plant defensins are members of the protein superfamily called the cis-defensins or CSαβ fold.[17] This superfamily includes arthropod defensins and fungal defensins (but not defensins found in mammals). It also includes several families of proteins not involved in the immune system, including plant S-locus 11 proteins involved in self-incompatibility during reproduction, and toxin proteins in scorpion venoms.[18][19]
Examples
The following plant proteins belong to this family:
- The flower-specific Nicotiana alata defensin (NaD1)
- Gamma-thionins from Triticum aestivum (wheat) endosperm (gamma-purothionins) and gamma-hordothionins from Hordeum vulgare (barley) are toxic to animal cells and inhibit protein synthesis in cell free systems.[15]
- A flower-specific thionin (FST) from Nicotiana tabacum (common tobacco).[20]
- Antifungal proteins (AFP) from the seeds of Brassicaceae species such as radish, mustard, turnip and Arabidopsis thaliana (thale cress).[21]
- Inhibitors of insect alpha-amylases from sorghum.[22]
- Probable protease inhibitor P322 from Solanum tuberosum (potato).
- A germination-related protein from Vigna unguiculata (cowpea).[23]
- Anther-specific protein SF18 from sunflower. SF18 is a protein that contains a gamma-thionin domain at its N-terminus and a proline-rich C-terminal domain.
- Glycine max (soybean) sulfur-rich protein SE60.[24]
- Vicia faba (broad bean) antibacterial peptides fabatin-1 and -2.
Databases
A database for antimicrobial peptides, including defensins is available: PhytAMP (http://phytamp.hammamilab.org).[25]
References
- 1 2 Parisi K, Shafee TM, Quimbar P, van der Weerden NL, Bleackley MR, Anderson MA (April 2019). "The evolution, function and mechanisms of action for plant defensins". Seminars in Cell & Developmental Biology. 88: 107–118. doi:10.1016/j.semcdb.2018.02.004. PMID 29432955. S2CID 3543741.
- ↑ Mendez E, Moreno A, Colilla F, Pelaez F, Limas GG, Mendez R, et al. (December 1990). "Primary structure and inhibition of protein synthesis in eukaryotic cell-free system of a novel thionin, gamma-hordothionin, from barley endosperm". European Journal of Biochemistry. 194 (2): 533–539. doi:10.1111/j.1432-1033.1990.tb15649.x. PMID 2176600.
- ↑ Colilla FJ, Rocher A, Mendez E (September 1990). "gamma-Purothionins: amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm". FEBS Letters. 270 (1–2): 191–194. doi:10.1016/0014-5793(90)81265-p. PMID 2226781. S2CID 9260786.
- ↑ Broekaert WF, Terras FR, Cammue BP, Osborn RW (August 1995). "Plant defensins: novel antimicrobial peptides as components of the host defense system". Plant Physiology. 108 (4): 1353–1358. doi:10.1104/pp.108.4.1353. PMC 157512. PMID 7659744.
- ↑ Terras FR, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, et al. (May 1995). "Small cysteine-rich antifungal proteins from radish: their role in host defense". The Plant Cell. 7 (5): 573–588. doi:10.1105/tpc.7.5.573. PMC 160805. PMID 7780308.
- ↑ Silverstein KA, Moskal WA, Wu HC, Underwood BA, Graham MA, Town CD, VandenBosch KA (July 2007). "Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants". The Plant Journal. 51 (2): 262–280. doi:10.1111/j.1365-313x.2007.03136.x. PMID 17565583.
- 1 2 Lay FT, Anderson MA (February 2005). "Defensins--components of the innate immune system in plants". Current Protein & Peptide Science. 6 (1): 85–101. doi:10.2174/1389203053027575. PMID 15638771.
- ↑ Cools TL, Struyfs C, Cammue BP, Thevissen K (April 2017). "Antifungal plant defensins: increased insight in their mode of action as a basis for their use to combat fungal infections". Future Microbiology. 12 (5): 441–454. doi:10.2217/fmb-2016-0181. PMID 28339295.
- ↑ Sathoff AE, Samac DA (May 2019). "Antibacterial Activity of Plant Defensins". Molecular Plant-Microbe Interactions. 32 (5): 507–514. doi:10.1094/mpmi-08-18-0229-cr. PMID 30501455.
- ↑ Pelegrini PB, Lay FT, Murad AM, Anderson MA, Franco OL (November 2008). "Novel insights on the mechanism of action of alpha-amylase inhibitors from the plant defensin family". Proteins. 73 (3): 719–729. doi:10.1002/prot.22086. PMID 18498107. S2CID 28378146.
- 1 2 Franco OL, Rigden DJ, Melo FR, Grossi-De-Sá MF (January 2002). "Plant alpha-amylase inhibitors and their interaction with insect alpha-amylases". European Journal of Biochemistry. 269 (2): 397–412. doi:10.1046/j.0014-2956.2001.02656.x. PMID 11856298.
- ↑ Pelegrini PB, Franco OL (November 2005). "Plant gamma-thionins: novel insights on the mechanism of action of a multi-functional class of defense proteins". The International Journal of Biochemistry & Cell Biology. 37 (11): 2239–2253. doi:10.1016/j.biocel.2005.06.011. PMID 16084753.
- ↑ Poon IK, Baxter AA, Lay FT, Mills GD, Adda CG, Payne JA, et al. (April 2014). "Phosphoinositide-mediated oligomerization of a defensin induces cell lysis". eLife. 3: e01808. doi:10.7554/ELIFE.01808. PMC 3968744. PMID 24692446.
- ↑ Baxter AA, Richter V, Lay FT, Poon IK, Adda CG, Veneer PK, et al. (June 2015). "The Tomato Defensin TPP3 Binds Phosphatidylinositol (4,5)-Bisphosphate via a Conserved Dimeric Cationic Grip Conformation To Mediate Cell Lysis". Molecular and Cellular Biology. 35 (11): 1964–1978. doi:10.1128/mcb.00282-15. PMC 4420927. PMID 25802281. S2CID 26373331.
- 1 2 Bruix M, Jiménez MA, Santoro J, González C, Colilla FJ, Méndez E, Rico M (January 1993). "Solution structure of gamma 1-H and gamma 1-P thionins from barley and wheat endosperm determined by 1H-NMR: a structural motif common to toxic arthropod proteins". Biochemistry. 32 (2): 715–724. doi:10.1021/bi00053a041. PMID 8380707.
- ↑ Janssen BJ, Schirra HJ, Lay FT, Anderson MA, Craik DJ (July 2003). "Structure of Petunia hybrida defensin 1, a novel plant defensin with five disulfide bonds". Biochemistry. 42 (27): 8214–8222. doi:10.1021/bi034379o. PMID 12846570.
- ↑ Dash TS, Shafee T, Harvey PJ, Zhang C, Peigneur S, Deuis JR, et al. (February 2019). "A Centipede Toxin Family Defines an Ancient Class of CSαβ Defensins". Structure. 27 (2): 315–326.e7. doi:10.1016/J.STR.2018.10.022. PMID 30554841.
- ↑ Shafee TM, Lay FT, Hulett MD, Anderson MA (September 2016). "The Defensins Consist of Two Independent, Convergent Protein Superfamilies". Molecular Biology and Evolution. 33 (9): 2345–2356. doi:10.1093/molbev/msw106. PMID 27297472.
- ↑ Shafee TM, Lay FT, Phan TK, Anderson MA, Hulett MD (February 2017). "Convergent evolution of defensin sequence, structure and function". Cellular and Molecular Life Sciences. 74 (4): 663–682. doi:10.1007/s00018-016-2344-5. PMID 27557668. S2CID 24741736.
- ↑ Gu Q, Kawata EE, Morse MJ, Wu HM, Cheung AY (July 1992). "A flower-specific cDNA encoding a novel thionin in tobacco". Molecular & General Genetics. 234 (1): 89–96. doi:10.1007/BF00272349. PMID 1495489. S2CID 32002467.
- ↑ Terras FR, Torrekens S, Van Leuven F, Osborn RW, Vanderleyden J, Cammue BP, Broekaert WF (February 1993). "A new family of basic cysteine-rich plant antifungal proteins from Brassicaceae species". FEBS Letters. 316 (3): 233–240. doi:10.1016/0014-5793(93)81299-F. PMID 8422949. S2CID 28420512.
- ↑ Bloch C, Richardson M (February 1991). "A new family of small (5 kDa) protein inhibitors of insect alpha-amylases from seeds or sorghum (Sorghum bicolar (L) Moench) have sequence homologies with wheat gamma-purothionins". FEBS Letters. 279 (1): 101–104. doi:10.1016/0014-5793(91)80261-Z. PMID 1995329. S2CID 84023901.
- ↑ Ishibashi N, Yamauchi D, Minamikawa T (July 1990). "Stored mRNA in cotyledons of Vigna unguiculata seeds: nucleotide sequence of cloned cDNA for a stored mRNA and induction of its synthesis by precocious germination". Plant Molecular Biology. 15 (1): 59–64. doi:10.1007/BF00017724. PMID 2103443. S2CID 13588960.
- ↑ Choi Y, Choi YD, Lee JS (February 1993). "Nucleotide sequence of a cDNA encoding a low molecular weight sulfur-rich protein in soybean seeds". Plant Physiology. 101 (2): 699–700. doi:10.1104/pp.101.2.699. PMC 160625. PMID 8278516.
- ↑ Hammami R, Ben Hamida J, Vergoten G, Fliss I (January 2009). "PhytAMP: a database dedicated to antimicrobial plant peptides". Nucleic Acids Research. 37 (Database issue): D963–D968. doi:10.1093/nar/gkn655. PMC 2686510. PMID 18836196.