 | |
| Names | |
|---|---|
| IUPAC name
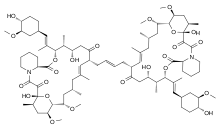
(1R,12S,13R,14S,18E,21S,23S,24R,25R,27R)-1,14-Dihydroxy-17-{(2E)-4-[(12S,13R,14S,17R,18E,21S,23S,24R,25R,27R)-14-hydroxy-12-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-propen-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-2,3,10,16-tetraoxo-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacos-18-en-17-yl]-2-buten-1-yl}-12-{(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-propen-2-yl}-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetrone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C86H134N2O23 | |
| Molar mass | 1564.009 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
FK1012 is a dimer consisting of two molecules of FK506 (tacrolimus) linked via their vinyl groups.[1] It is used as a research tool in chemically induced dimerization applications. It is used in pharmacology to act as a mediator in the formation of FK506 dimer.[2] FK506 binding proteins (FKBPs) do not normally form dimers but can be caused to dimerize in the presence of FK1012. Genetically engineered proteins based on FKBPs can be used to manipulate protein localization, signaling pathways and protein activation.[3]
References
- ↑ Steven T. Diver; Stuart L. Schreiber (1997). "Single-step syntheses of cell permeable protein dimerizers that activate signal transduction and gene expression". J. Am. Chem. Soc. 119 (22): 5106–5109. doi:10.1021/ja963891c.
- ↑ Blau, C. Anthony; Peterson, Kenneth R.; Drachman, Jonathan G.; Spencer, David M. (1997). "A Proliferation Switch for Genetically Modified Cells". Proceedings of the National Academy of Sciences of the United States of America. 94 (7): 3076–3081. ISSN 0027-8424.
- ↑ Fegan, A; White, B; Carlson, JC; Wagner, CR (Jun 9, 2010). "Chemically controlled protein assembly: techniques and applications". Chemical Reviews. 110 (6): 3315–36. doi:10.1021/cr8002888. PMID 20353181.
Further reading
- Otto, K. G.; Jin, L; Spencer, D. M.; Blau, C. A. (2001). "Cell proliferation through forced engagement of c-Kit and Flt-3". Blood. 97 (11): 3662–4. doi:10.1182/blood.V97.11.3662. PMID 11369667.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.