 | |
| Clinical data | |
|---|---|
| Other names | 19-Nor-4,9(10)-androstadienedione; Estradienedione, 19-Norandrosta-4,9(10)-diene-3,17-dione; Estra-4,9(10)-diene-3,17-dione |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.112.341 |
| Chemical and physical data | |
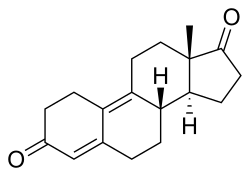
| Formula | C18H22O2 |
| Molar mass | 270.372 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
Dienedione, also known as estra-4,9-diene-3,17-dione, is a synthetic, orally active anabolic-androgenic steroid (AAS) of the 19-nortestosterone group that was never introduced for medical use. It is thought to be a prohormone of dienolone.[1] The drug became a controlled substance in the US on January 4, 2010,[2] and is classified as a Schedule III anabolic steroid under the United States Controlled Substances Act. Previous to this, it was sold as a bodybuilding supplement within the United States, and often mistakenly marketed as a prohormone for trenbolone, a veterinary steroid. Prior to its scheduling, it was part of a number of supplements that were seized during FDA enforcement of Bodybuilding.com for selling unapproved new drugs.[3] The actual active metabolite, dienolone, is almost identical to trenbolone structurally, but lacks the C11 double bond.
See also
References
- ↑ "Rules - 2009 - Final Rule: Classification of Three Steroids as Schedule III Anabolic Steroids Under the Controlled Substances Act". www.deadiversion.usdoj.gov. Retrieved 1 June 2017.
- ↑ "Rules - 2009 - Final Rule: Classification of Three Steroids as Schedule III Anabolic Steroids Under the Controlled Substances Act". www.deadiversion.usdoj.gov. Retrieved 1 June 2017.
- ↑ Office of Regulatory Affairs. "Enforcement Reports - Enforcement Report for July 7, 2010". www.fda.gov. Retrieved 1 June 2017.