 | |
| Clinical data | |
|---|---|
| Pronunciation | peg val' i ase |
| Trade names | Palynziq |
| Other names | Pegvaliase-pqpz; PEG-PAL; RAvPAL-PEG |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618057 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
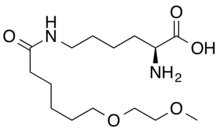
| Formula | C15H30N2O5 |
| Molar mass | 318.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pegvaliase, sold under the brand name Palynziq, is a medication for the treatment of the genetic disease phenylketonuria.[4][5] Chemically, it is a pegylated derivative of the enzyme phenylalanine ammonia-lyase that metabolizes phenylalanine to reduce its blood levels.[6]
It was approved by the Food and Drug Administration for use in the United States in 2018.[4] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[7]
References
- 1 2 "Palynziq". Therapeutic Goods Administration (TGA). 23 July 2021. Archived from the original on 5 September 2021. Retrieved 5 September 2021.
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Archived from the original on 3 April 2022. Retrieved 13 May 2022.
- ↑ "Palynziq Product information". Health Canada. 25 April 2012. Archived from the original on 29 June 2022. Retrieved 29 June 2022.
- 1 2 "FDA approves a new treatment for PKU, a rare and serious genetic disease" (Press release). Food and Drug Administration. May 24, 2018. Archived from the original on July 25, 2021. Retrieved October 12, 2020.
- ↑ Mahan KC, Gandhi MA, Anand S (April 2019). "Pegvaliase: a novel treatment option for adults with phenylketonuria". Current Medical Research and Opinion. 35 (4): 647–651. doi:10.1080/03007995.2018.1528215. PMID 30247930. S2CID 52813510.
- ↑ "Palynziq". BioMarin Pharmaceutica. Archived from the original on 2020-06-26. Retrieved 2018-06-21.
- ↑ New Drug Therapy Approvals 2018 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2019. Archived from the original on 17 September 2020. Retrieved 16 September 2020.
External links
- "Pegvaliase". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.