 | |
| Clinical data | |
|---|---|
| Other names | 2,5-Dimethoxy-4-propylamphetamine |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H23NO2 |
| Molar mass | 237.343 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
2,5-Dimethoxy-4-propylamphetamine (DOPR) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL (Phenethylamines i Have Known And Loved). Shulgin described DOPR as a "heavy duty psychedelic", complete with alterations of the thought process and visual distortion.[1] Very little data exists about the pharmacological properties, metabolism, and toxicity of DOPR.
The alternative structural isomer DOIP, with a 4-isopropyl substitution, is also known but is around ten times weaker than DOPR, with an active dose of some 20–30 mg (as compared to 2–5 mg for DOPR).[1]
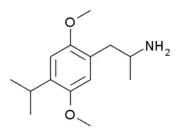
Structure of DOIP
See also
References
- 1 2 Shulgin A, Shulgin A (September 1991). PiHKAL: A Chemical Love Story. United States: Transform Press. p. 978. ISBN 0-9630096-0-5.
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.